HIPPO AUSTRALIA STUDY HUB
HIPPO - Hernias, Pathway and Planetary Outcomes for Inguinal Hernia Surgery
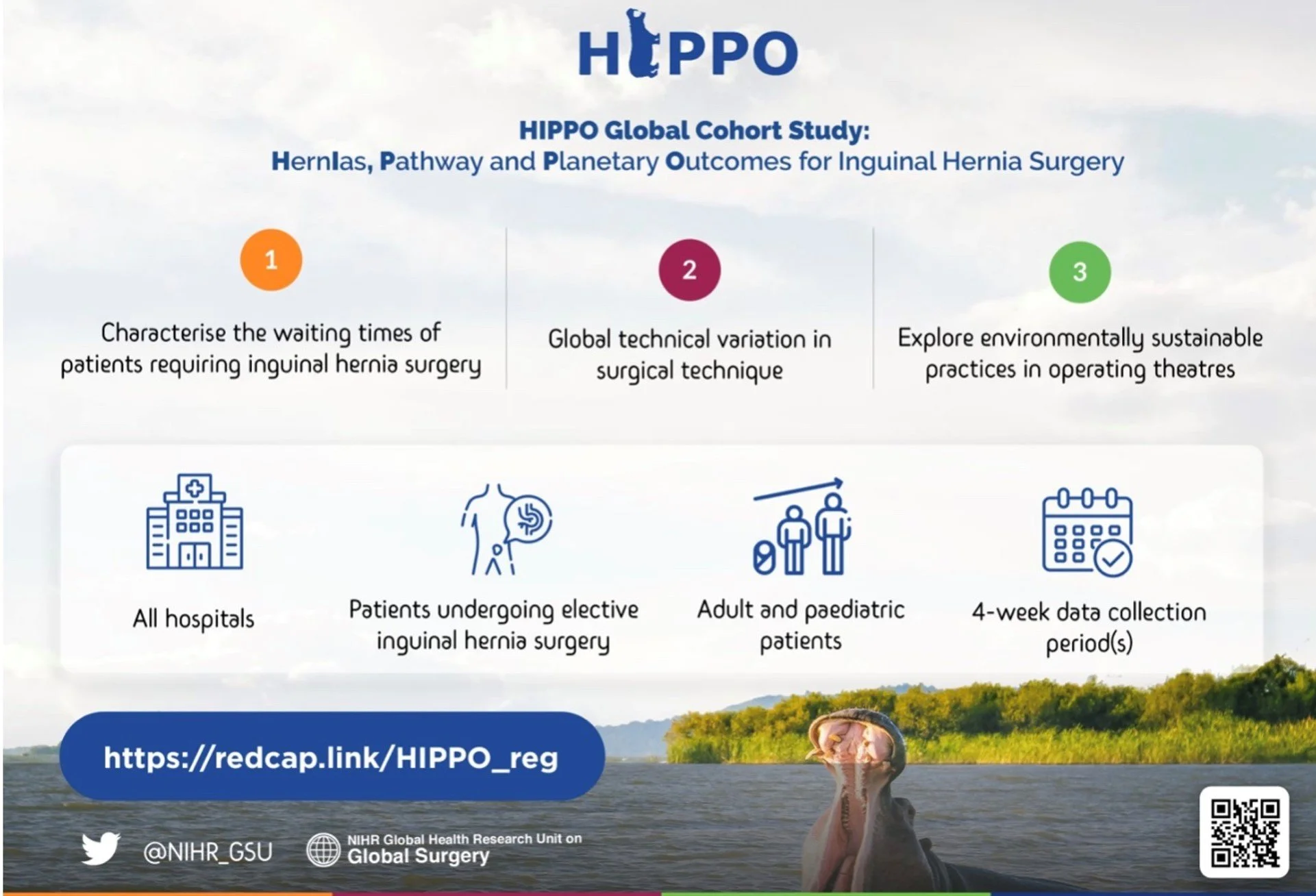
What is the HiPPO Study?
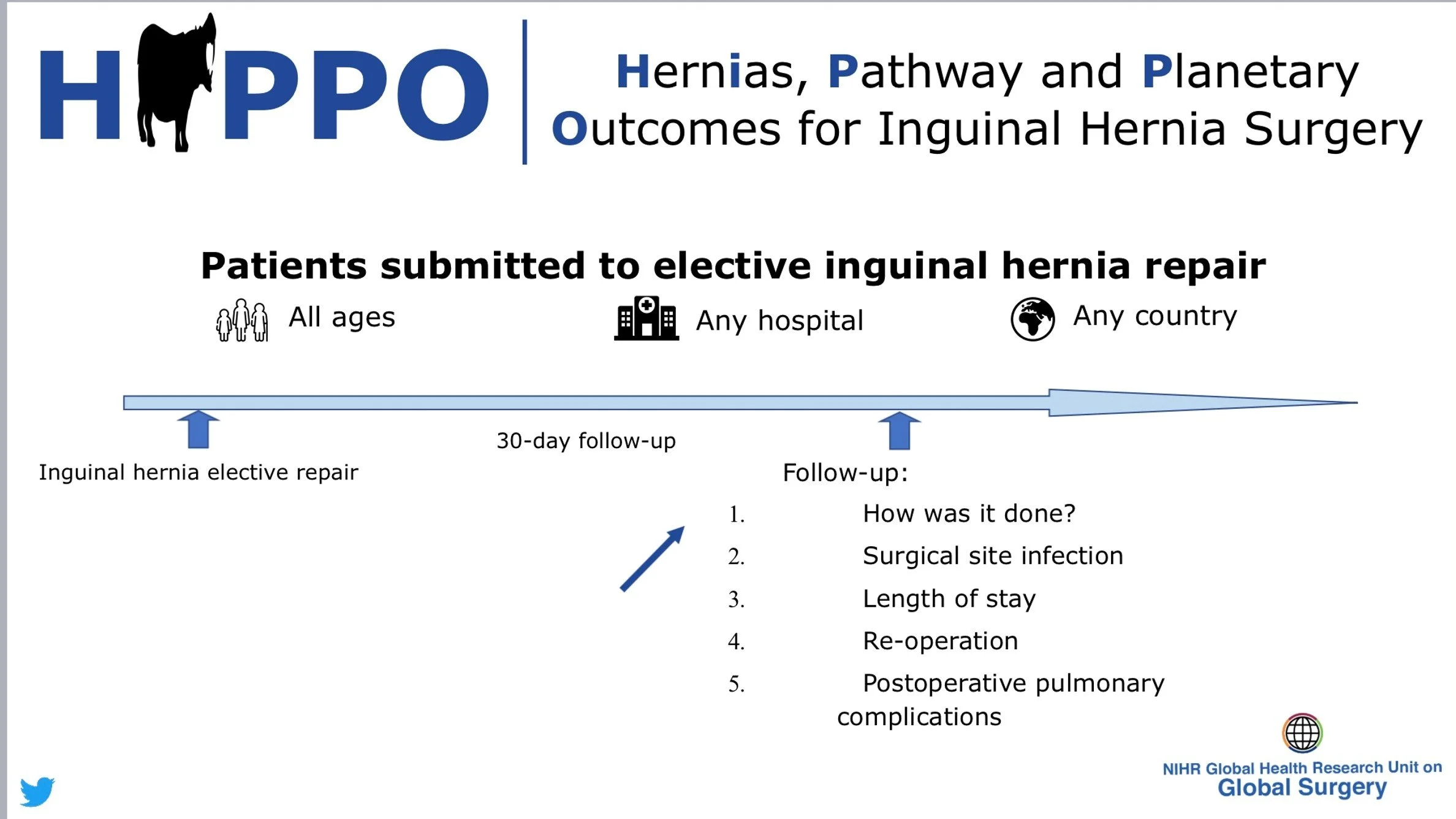
HiPPO is an international, multi-centre, prospective observational cohort study investigating the management of patients undergoing inguinal hernia repair. The aims are to characterise the global backlog for elective surgery, understand the technique, training and operative variation in hernia repair, and finally to explore environmentally sustainable practices in operating theatres. The study is being delivered by the NIHR Global Health Research Unit on Global Surgery, and will be facilitated in Australia by STORCC. Through facilitating the HiPPO Study in Australiia we aim to build collaborative research capacity amongst medical students and junior doctors.
ALL HOSPITALS | GENERAL & PAEDIATRIC SURGERY |
FOUR-WEEK DATA COLLECTION
Who is Involved?
STORCC invites all medical students in their clinical years, junior doctors, surgical registrars and consultants to become HiPPO collaborators.
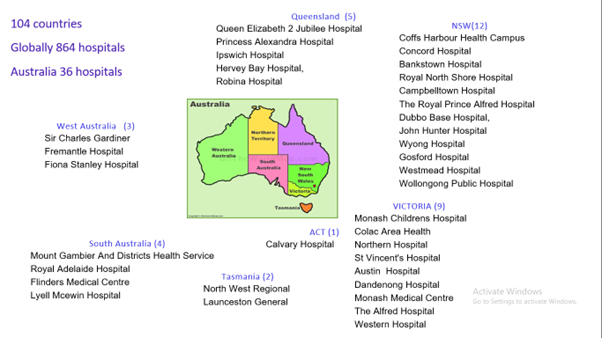
All collaborators will be included as PubMed-citable co-authors on resulting publications. All hospitals with adults presenting for emergency or elective inguinal hernia surgery can participate in this study.
If you have any further queries please email us at: hippostudyaus@gmail.com
AUSTRALIAN INVESTIGATORS HOW TO BE INVOLVED
Australian National Ethics has been approved via a greater than low risk pathway with the Hunter New England HREC
IMPORTANT NOTE: Australian sites, the Hunter New England Human Research Ethics Committee has approved a waiver of consent under section 2.3.10 of the National Statement of Ethical Conduct in Human Research, 2007 (updated 2018) for the HIPPO study protocol.
IF YOU HAVE NOT YET REGISTERED AS AN AUSTRALIAN COLLABORATOR OR HOSPITAL LEAD FOR HIPPO:
Follow this link to the Globalsurg HIPPO website, to can register your interest here..
Check if there is already a hospital lead registered for your site:
Hospital Lead already registered - Register as a collaborator and please contact your local hospital lead.
No Hospital Lead registered - Register as the hospital lead - please contact the Australian HIPPO team
hippostudyaus@gmail.com
IF YOU ARE A CONFIRMED AUSTRALIAN HOSPITAL SITE LEAD:
Once you have registered your site with the Globalsurg HIPPO team
Your hospital site needs to be added to the national ethics approval AND EACH site needs to provide following information to the Australian team. hippostudyaus@gmail.com
Each Australian site then needs a supervisting consultant as the PI for the ethics approval process - so if hospital leads have not yet identified a local consultant supervisor you now need to do this as we need to add the following information
This is the detailed descriptions of the data required to complete this process in REGIS e-fields:
· For NSW and ACT sites; Project Centre & Project Site (and for non NSW and ACT sites; State and Project site)
· For non-NSW and ACT sites; Is the site a Public Health Organisation or Private Hospital?
· All states; Principal Investigator (PI) name and email address (the account linked to their REGIS account for every NSW and ACT PI – but this is not necessary for non-REGIS users from other states)
· All states; PI Contact phone (mobile preferred), PI Position
· All states, PI Employer (and for non-NSW and ACT sites, the employee number).
· For NSW and ACT sites; PI Department
· All states; Describe the research activities this person (PI) will be responsible for at this site (we can enter a standard answer to this question for all sites) -
· All states; Describe the person’s (PIs) relevant expertise relevant to this research activity they will undertake at this site
· For NSW and ACT sites; Is the new investigator (PI) an employee of NSW Health
· All states; Is the new investigator (PI) a student?
· All states; Are you adding new site to a single centre study (we will answer this as no for all sites)
· All states; Do any members of the research team (including persons not listed in this application), have any financial or non-financial interests related to this research?
· All states; We require a copy of the PI’s CV to be uploaded into REGIS (a summarised version is fine
HiPPO Study Documents:
Please see the study-wide HiPPO Study Hub for the Australian specific study documents (protocol and data collection form). Please note Australian sites have a waiver of consent and therefore the patient consent form is not required.
IMPORTANT NOTE: Australian sites, the Hunter New England Human Research Ethics Committee has approved a waiver of consent under section 2.3.10 of the National Statement of Ethical Conduct in Human Research, 2007 (updated 2018) for the HIPPO study protocol.
Click the following links: